On 27 and 28 October 2025, ECHA organized a stakeholders webinar workshop on the Drinking Water Directive (DWD). The workshop covered topics such as legal obligations and data requirements for notifications of intention and applications under the Drinking Water Directive, alongside IT tools, submission systems, and available support material. The DWD is the EU’s legislative instrument meant to ensure that water intended for human consumption is wholesome and clean, and that consumers have access to safe, high-quality drinking water. 
On day one, Bart Leemans – DG Environment at the EC presented the Implementation of EU hygiene requirements for materials in contact with drinking water: an overview by the European Commission.
A recast on the DWD, to improve the health protection:
- Update on the safety standards at the point of compliance, WHO recommendations
- Introduction on DWD “watch-list”, to monitor substances of emerging concern
- Introduction of risk based approach covering the entire water supply chain
- Access to water, obligation for MS to improve and maintain access to safe drinking water for all
- Provisions on materials in contact with drinking water, Article 11 of DWD, to be used in new installations or in the case of repair works or reconstruction, in existing installations
- More transparency for consumers
Panos Zarogiannis – DWD Teamleader at EC presented the DWD European Positive List.
The Positive Lists identify starting substances, compositions, or constituents authorised for use in manufacturing materials or products that contact drinking water. There are four material categories covered with 2042 entries for:
The Positive Lists identify starting substances, compositions, or constituents authorised for use in manufacturing materials or products that contact drinking water. There are four material categories covered with 2042 entries for:
Annex I – Organic materials with 1643 entries
Annex II – Metallic materials and their alloys with 50 entries
Annex III – Cementitious materials with 335 entries
Annex IV – Enamel, ceramic, or other inorganic materials with 14 entries
The positive lists are based on national positive lists submitted by MS via ECHA in July 2021.
The European Commission adopted implementing decisions and delegated regulations on 23 April 2024 to establish the hygiene requirements and positive lists.
The new European Positive Lists apply from 31 December 2026 for new installations and materials/products in contact with drinking water. Until then, national provisions continue to apply. For substances which MS approves between 13 July and 31 December 2026, a limited transitional period applies until 31 December 2032. After the transition period, only materials/products incorporating starting substances/compositions/constituents listed on the European Positive Lists may be placed on the market for drinking‐water contact use.
Each entry in the positive lists has a unique EUPL chemical identifier of the approved substance, composition, an expiry date, specific conditions of use, and migration‐limit values.
Panos Zarogiannis – DWD Team Leader at EC presented the DWD Notification of Intention and DWD applications.
Before submitting a full application for inclusion in a positive list, an economic operator must submit a notification of intention to ECHA. Guidance is provided by ECHA.
The notification of intention must be prepared using the IUCLID system (International Uniform Chemical Information Database) and should include preliminary information such as identity of substance, manufacturer/importer, intended uses, available data, and prioritisation.
After the notification of intention, the full DWD application dossier must be submitted, providing all required data: hazard assessment, migration testing, conditions of use, any risk mitigation measures, etc. This is outlined in ECHA DWD guidance (Volumes I–IV), which covers methodologies for testing, acceptance, scope, and notification contents, and ECHA’s NoI – IUCLID manual.
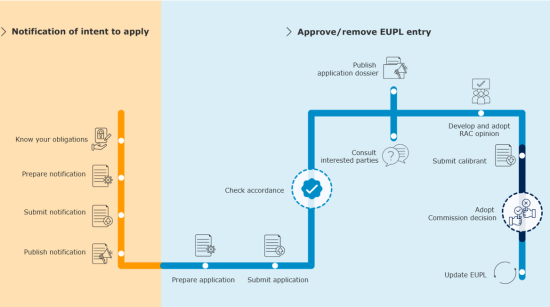
The implementing decision provides that conformity assessment, marking (an EU marking for drinking water contact products), and product monitoring will apply.
Strategic actions for stakeholders
• Identify all materials/products in contact with drinking water in your portfolio and map which starting substances/compositions/constituents are used.
• Check whether these substances/compositions/constituents are already on the national positive list (for now) or likely to be on the European Positive List.
• If not yet listed, consider submitting a notification of intention (2025) and plan for full application (2026) to secure inclusion.
• For new product development, ensure from the design stage that materials meet the upcoming European standards (lead content, migration values, hygienic design).
• Engage with conformity assessment bodies and certification to align with upcoming EU marking and documentation obligations.
• Monitor expiry dates of entries on the positive lists: substances may require re-evaluation; if not supported, they may be removed.
• Monitor the cost of dossier preparation, testing, submission, and certification must be factored into business planning.
• Keep an eye on amendments: The positive lists will be reviewed, substances will expire, and stakeholders may apply for additions; staying up to date is critical.
On day 2, workshop demonstrations were given on how to apply for:
NoI demonstration Part I: Preparing for making a DWD notification of intention.
NoI demonstration Part II: Creation of the IUCLID NoI dossier.
NoI demonstration Part III: Submission of the IUCLID notification of intention dossier.
NoI demonstration Part IV: Publication of the IUCLID notification of intention dossier.
More information on https://www.echa.europa.eu/dwd-processes
The revised Drinking Water Directive elevates expectations across the entire drinking-water supply chain: from abstractors, through treatment and distribution, materials in contact with water, to the end-consumer’s tap. The given tight timeframes, means that preparation must start now.
For stakeholders, the take-home is: move from “waiting for regulation” to actively preparing: audit your systems, materials, monitoring, communications; build your roadmap; engage your supply-chain; prepare your reporting/IT systems; engage consumers and ensure you stay ahead of the curve.
Sandy Van den Broeck -ESG Director
